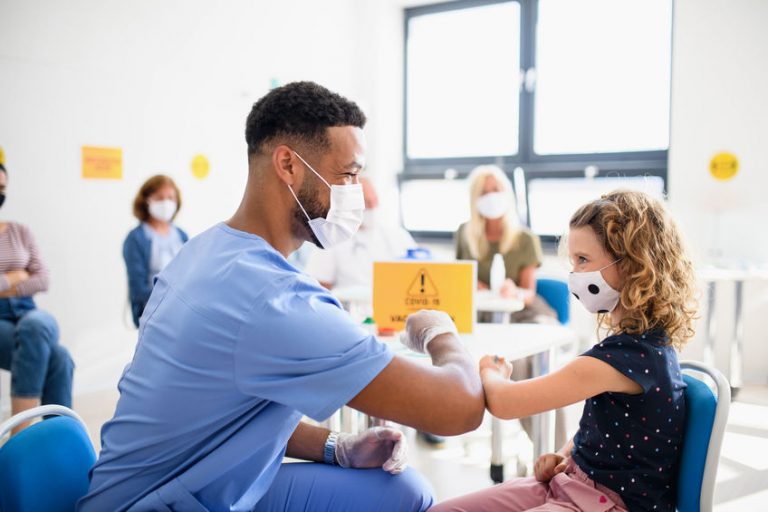
The authorization by the Food & Drug Administration of the Pfizer vaccine for children ages 12 to 16 years and the strong recommendation by the Centers for Disease Control & Prevention (CDC) to administer the vaccine are major landmarks in the history of slowing the COVID-19 pandemic. These important events likely raise the issue for some as to whether children 12-16 years with trisomy 18, 13, and related disorders should be vaccinated. Currently only the Pfizer vaccine is approved for children 12-18 years.
My Recommendations are the following:
- Consult your child’s or adult’s physician or other care provider to discuss the benefits, risks, and side effects of the vaccine
- Inquire about any special circumstances surrounding your child’s personal medical history, for example, immunocompromised children
- Review the CDC’s informative website, which includes Frequently-Asked Questions, www.cdc.gov
My own opinion is straightforward: I favor vaccinations for children with genetic syndromes, including SOFT conditions; this was my opinion earlier this year when asked about adults with trisomy 18, 13, and related disorders. As we know persons with trisomy 18 and 13 often have pulmonary hypertension, obstructive sleep apnea, & underlying cardiac defects, which place them at a significantly higher risk for COVID-related complications.
Importantly the Pfizer and Moderna vaccines, previously authorized for adults, are not experimental (a concern appropriately raised by some). Papers published in leading scientific journals have shown the vaccines to be highly effective in preventing COVID-19 and in preventing a serious course in those who acquire the infection. Additionally development of these vaccines followed nicely from work done for years at the National Institutes of Health on other coronaviruses, allowing the relatively rapid process to clinical trial completion. Follow-up studies by the CDC on the millions of persons who have received the vaccines since late 2020 document even higher effectiveness than the original studies. The benefits of the vaccines far outweigh the risks and the side effects of the vaccine.
One other specific point about vaccinations related to SOFT conditions: there are 2 sources that indicate that children with trisomy 18 and 13 do NOT have adverse reactions related to traditional immunizations (such as polio and MMR): the study by Bonnie Baty and myself in 1994 ( available on the www.trisomy.org site under the Medical Professional dropdown, Literature/Care & Management) and data collected when families registered as members of SOFT (unpublished).
Respectfully submitted,
John C Carey, MD, MPH
Chair, Medical & Scientific Advisory Board, SOFT































Recent Comments